This simulation compares the rate of energy transfer from heated metal blocks to ice water in two beakers, each under its own set of initial conditions. When the green "Start" button is clicked, one or more hot metal blocks drop into beakers containing ice water, and the block temperature and/or the mass of ice melted is plotted versus time by selecting the appropriate radio button. The number of blocks and their properties are adjusted with sliders. Checking "Add stir bar" increases the heat transfer rate. Comparisons can be made by choosing different conditions for beakers 1 and 2. To change the initial conditions for another experiment, first press the Reset button. This simulation assumes perfect mixing, no temperature gradient in the metal blocks, and that all energy transferred from the blocks goes into melting the ice. Once the ice fully melts, the energy goes into raising the water temperature.
This simulation is a lumped capacitance method of examining transient conduction. It assumes that temperature gradients within the block and water are negligible. This may not be realistic. The overall energy balance on the system is
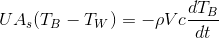
where 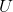 is the overall
heat transfer coefficient,
is the overall
heat transfer coefficient,
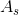 is the surface area for heat transfer,
is the surface area for heat transfer,
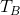 and
and 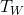 are the temperature of the block and water, respectively,
are the temperature of the block and water, respectively,
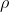 and
and
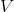 are the block density and volume,
are the block density and volume, 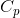 is the block heat capacity, and
is the block heat capacity, and
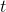 is time.
is time.
If 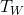 is constant (i.e., there is sufficient ice to keep
the water at 0°C) and we let
the block's initial temperature be
is constant (i.e., there is sufficient ice to keep
the water at 0°C) and we let
the block's initial temperature be 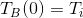 ,
the solution to this ordinary differential equation becomes:
,
the solution to this ordinary differential equation becomes:
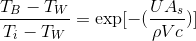
(or rearranging gives us)
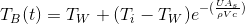
The total amount of ice melted can then be calculated:
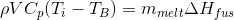
where 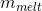 is the
mass of ice melted, and
is the
mass of ice melted, and 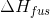 is
ice's heat of fusion.
is
ice's heat of fusion.
Until the ice fully melts, energy transfer from the block will not affect the temperature of the water.
Once all the ice melts, the water temperature will rise.
This simulation was created in the Department of Chemical and Biological Engineering, at University of Colorado Boulder for LearnChemE.com by Neil Hendren under the direction of Professor John L. Falconer. It is a JavaScript/HTML5 implementation of a simulation by Matt Koppenhaver, Dr. Michael Prince, Dr. Margot Vigeant, and Emily Ehrenberger at Bucknell University1. As of 1-24-2023, the original version is accessible here. Address any questions or comments to learncheme@gmail.com. All of our simulations are open source, and are available on our LearnChemE Github repository.
If this simulation is too big for your screen, zoom out using  +
+  on Mac or
on Mac or  +
+  on Windows. To zoom in,
use
on Windows. To zoom in,
use  +
+  on Mac or
on Mac or  +
+  on Windows.
on Windows.
References:
1Prince, M., Vigeant, M., Nottis, K., Repairing student misconceptions in heat transfer using inquiry-based activities, Chemical Engineering Education 50(1), 52 (2016).